LOPTAN-HZ® – Losartan Potassium BP & Hydrochlorothiazide BP
Composition:
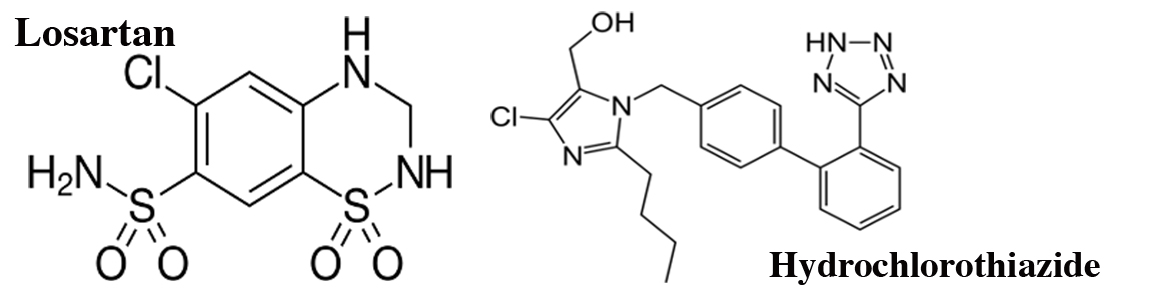
Loptan-HZ 50 Tablet: Each film coated tablet contains Losartan Potassium USP 50 mg and Hydrochlorothiazide BP 12.5 mg.
Loptan-HZ 100 Tablet: Each film coated tablet contains Losartan Potassium USP 100 mg and Hydrochlorothiazide BP 25 mg.
Pharmacology:
Angiotensin II formed from angiotensin I in a reaction catalyzed by angiotensin converting enzyme (ACE), is a potent vasoconstrictor, the primary vasoactive hormone of the renin-angiotensin system and an important component in the pathophysiology of hypertension. It also stimulates aldosterone secretion by the adrenal cortex. Losartan and its principal active metabolite block the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor found in many tissues, (e.g. vascular smooth muscle, adrenal gland). In vitro binding studies indicate that Losartan is a reversible, competitive inhibitor of the AT1 receptor. Neither Losartan nor its active metabolite inhibits ACE (kinase II, the enzyme that converts angiotensin I to angiotensin II and degrades bradykinin); nor do they bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation.
Hydrochlorothiazide is a thiazide diuretic. Thiazides affect the renal tubular mechanisms of electrolyte reabsorption, directly increasing excretion of sodium and chloride in approximately equivalent amounts. Indirectly, the diuretic action of Hydrochlorothiazide reduces plasma volume, with consequent increases in plasma renin activity, increases in aldosterone secretion, increases in urinary potassium loss, and decreases in serum potassium. The renin-aldosterone link is mediated by angiotensin II, so co-administration of an angiotensin II receptor antagonist tends to reverse the potassium loss associated with these diuretics.
Indications:
Loptan-HZ is indicated for the treatment of hypertension. It is also indicated to reduce the risk of stroke in patients with hypertension and left ventricular hypertrophy.
Dosage & Administration:
Hypertension:
• Usual starting dose: 50/12.5 mg once daily. Titrate as needed to a maximum dose of 100/25 mg. Hypertensive Patients with Left Ventricular Hypertrophy:
• Not controlled on monotherapy: Initiate with 50/12.5 mg. Titrate as needed to a maximum of 100/25 mg.
Dosing must be individualised. The usual starting dose of Losartan is 50 mg once daily, with 25 mg recommended for patients with intravascular volume depletion (e.g., patients treated with diuretics) and patients with a history of hepatic impairment. Losartan can be administered once or twice daily at total daily doses of 25 to 100 mg. If the antihypertensive effect measured trough using once a day dosing is inadequate, a twice a day regimen at the same total daily dose or an increase in dose may give a more satisfactory response.
Hydrochlorothiazide is effective in doses of 12.5 to 50 mg once daily and can be given at doses of 12.5 to 25 mg. To minimize dose independent side effects, it is usually appropriate to begin combination therapy only after a patient has failed to achieve the desired effect with monotherapy.
Contraindications:
Contraindicated to patients with hypersensitivity to any component of this product.
Precautions:
Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be performed at appropriate intervals. All patients receiving thiazide therapy should be observed for clinical signs of fluid or electrolyte imbalance: hyponatraemia, hypochloremic alkalosis, and hypokalaemia. Because Losartan decreases uric acid, Losartan in combination with Hydrochlorothiazide attenuates the diuretic-induced hyperuricaemia. In diabetic patients dosage adjustments of Insulin or oral hypoglycemic agents may be required. Hyperglycemia may occur with thiazide diuretics. The antihypertensive effects of the drug may be enhanced in the post-sympathectomy patient. If progressive renal impairment becomes evident, consider withholding or discontinuing diuretic therapy. Thiazides have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesaemia. Thiazides may decrease urinary calcium excretion. Increases in cholesterol and triglyceride levels may be associated with thiazide diuretic therapy
Side Effects:
Generally this product is well tolerated. However, few side effects including abdominal pain, swelling, back pain, dizziness, rash and cough may occur in rare cases.
Use in Pregnancy & Lactation:
When pregnancy is detected, Losartan & Hydrochlorothiazide should be discontinued as soon as possible. It is not known whether Losartan is excreted in human milk. Thiazides appear in human milk. Because of the potential for adverse effects on the nursing infant, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Drug Interactions:
Losartan Potassium
No significant drug-drug pharmacokinetic interactions have been found in interaction studies with Hydrochlorothiazide, Digoxin, Warfarin, Cimetidine and Phenobarbital. As with other drugs that block angiotensin II or its effects, concomitant use of potassium-sparing diuretics (e.g. Spironolactone, Triamterene, Amiloride), potassium supplements, or salt substitutes containing potassium may lead to increase in serum potassium. As with other antihypertensive agents, the antihypertensive effect of Losartan may be blunted by the non-steroidal anti-inflammatory drug I ndomethacin.
Hydrochlorothiazide
When administered concurrently, the following drugs may interact with Thiazide diuretics: alcohol, barbiturates, or narcotics – potentiation of orthostatic hypotension may occur.
Antidiabetic drugs (oral agents and Insulin) – dosage adjustment of the antidiabetic drug may be required.
Other antihypertensive drugs – additive effect or potentiation.
Cholestyramine and colestipol resins – absorption of Hydrochlorothiazide is impaired in the presence of anionic exchange resins.
Over Dosage:
Losartan Potassium
Limited data are available in regard to over dosage in humans. The most likely manifestation of over dosage would be hypotension and tachycardia; bradycardia could occur from parasympa¬thetic (vagal) stimulation of symptomatic hypotension should occur, supportive treatment should be instituted. Neither losartan nor its metabolite can be removed by hemodialysis.
Hydrochlorothiazide
The most common signs and symptoms observed are those caused by electrolyte depletion (hypokalemia, hypochloremia, and dehydration resulting from excessive diuresis).
Storage:
Store in a cool, dry place & below 30° C. Protected from light . Keep out of the reach of children.
Packing:
Loptan-HZ 50 Tablet: Each box contains 3 x 10’s film coated tablets in Alu-Alu blister pack. Loptan-HZ 100 Tablet: Each box contains 3 x 10’s film coated tablets in Alu-Alu blister pack.