PROBET CREAM® – Clobetasol Propionate BP
Clobetasol Propionate BP 0.05% w/w
DESCRIPTION
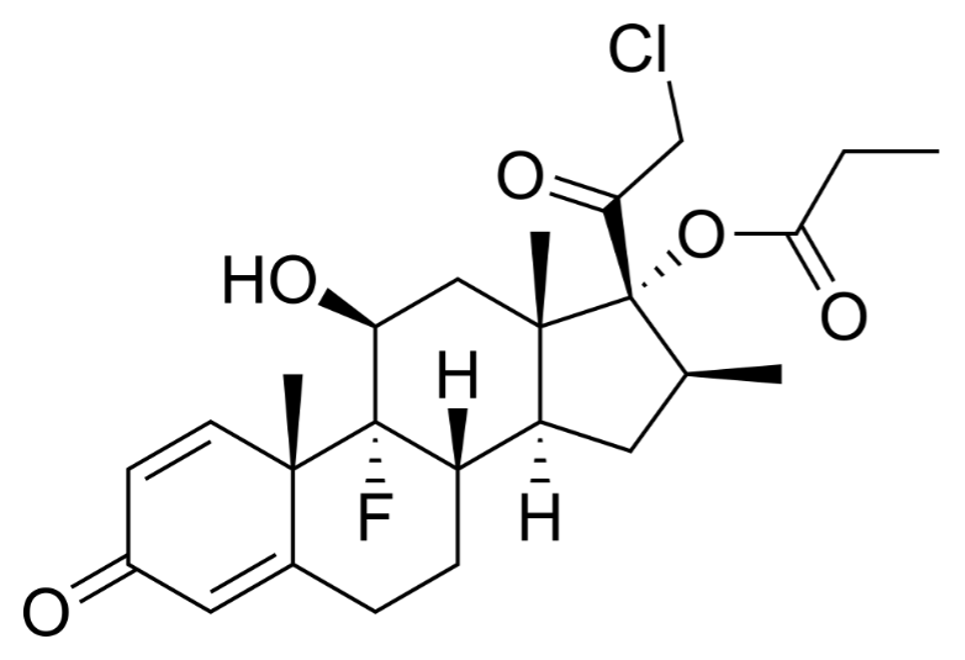
PROBET® Cream contains the compound Clobetasol Propionate BP an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree of mineralocorticoid activity.
COMPOSITION
Each gram cream contains Clobetasol Propionate BP 0.5 mg.
PHARMACOLOGY
PROBET® has both local anti-inflammatory and immunosuppressive activity. Thus PROBET® inhibits the adherence of neutrophils and monocyte-macrophages to the capillary endothelial cells of the inflammed area. The drug blocks the effect of macrophage migration inhibitory factor and decreases the activation of plasminogen to plasmin. PROBET® also reduces the number of human leukocyte antigen-DR-qT6+. By inhibition of phospholipase A, activity via formation of lipocortin. Clobetasol reduces the formation of prostaglandins and leukotrienes in the local tissues.
INDICATIONS
• Initial control of all forms of hyperacute eczema in all age groups chronic hyperkeratotic eczema of the hands and feet and patches of chronic lichen simplex
• Chronic hyperkeratotic psoriasis of any area of the body
• Severe acute photosensitivity
• Acute contact dermatitis Hypertrophic lichen planus
• Localized bullous disorders
• Keloid scarring
• Pretibial myxedema
• Vitiligo
• Suppression of reactions after cryotherapy.
DOSAGE AND APPLICATION
PROBET® should be applied with gentle rubbing to the affected skin areas twice daily. Once absorbed through the skin, topical corticosteroids enter pharmacokinetic pathways similarly to systemically administered corticosteroids. For acute dermatitis twice daily application may be appropriate for short periods of time e.g. 1-2 weeks, although evidence now suggests that once daily application is sufficient. Long term treatment should probably be limited to intermittent plus therapy as skin atrophy has been shown to persist for 3 days after single application.
Dosage should not normally exceed 50 g per week and the duration of a course should not be more than 4 weeks. The potency can be further enhanced by the use of occlusive dressings.
SIDE EFFECTS
The use of large amounts of cream for a long period of time over very large areas of the body can lead to produce adrenal suppression, cushing syndrome; hypercorticism; pigmentation; atrophic changes, such as thinning, striae; etc.
CONTRAINDICATIONS
• Cutaneous infections such as impetigo, tines corporis and herpes simplex
• Infestations such as scabies Acne vulgaris
• Children under 1 year
• Hypersensitivity
• Rosacea
• Gravitational ulceration
• Perioral dermatitis.
PRECAUTIONS
Avoid long-term therapy particularly in children, in whom adrenal suppression occurs readily. If PROBET® is required for use in children, it is recommended that the treatment should be weekly reviewed. Avoid prolonged application to face because the face than other area of the body exhibits more atrophic changes with potent corticosteroids.
Discontinue the use if there is spread of infection. Avoid contact with eyes.
USE IN PREGNANCY
PROBET® should be avoided in pregnant women.
USE IN LACTATION
Mothers Using large amounts of the drug should be aware potential excretion in milk.
PAEDIATRIC USE
The drug may be used in pediatric patients in appropriate doses, but large quantities for prolonged period should be avoided. It is contraindicated in children under 1 year.
DRUG INTERACTIONS
No hazardous interactions have been found.
INFORMATION FOR PATIENTS
• This medication is to be used as directed by the physician and should not be used longer than the prescribed time period. It is for external use only. Avoid contact with eyes.
• The treated skin area should not be bandaged or otherwise covered or warped so as to be occlusive.
• This medication should not be use for any disorder other than that for which it was prescribed.
• Patients should report any signs of local adverse reactions to the physicians.
STORAGE
• Keep in a cool (below 30° C) & dry place.
• Close the mouth of tube well after each application.
• Do not freeze.
PACKING
Collapsible aluminium tube containing 10 g cream.