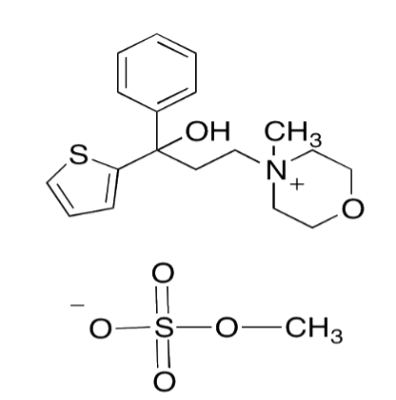
TYVAS ®–Tiemonium Methylsulphate INN
COMPOSITION
Available in 2 different Dosage forms:
50 mg Tablet: Each film coated tablet contains 50 mg Tiemonium Methylsulphate INN.
Injection: Each 2 ml ampoule contains 5 mg Tiemonium Methylsulphate INN.
PHARMACOLOGY
Tiemonium Methylsulphate is a quarternary ammonium antimuscarinic agent with spasmolytic (antispasmodic) and parasympatholytic (anticholinergic) effects and is used in the relief of visceral spasms (of the intestine, biliary system, uterus and urinary bladder in gastrointestinal, biliary, urinary and gynecological issues)
INDICATION
Tiemonium Methylsulphate is an antispasmodic drug. It reduces muscle spasms of the intestine, biliary system, uterus & urinary bladder. It is indicated for the pain associated with gastrointestinal & biliary disease. It is also indicated for urological & gynecological pain such as gastroenteritis, diarrhea, dysentery, biliary colic, enterocolitis, cholecystitis, colonopathies, mild cystitis & spasmodic dysmenorrhea etc.
DOSAGE AND ADMINISTRATION
Recommended oral dose of Tiemonium Methylsulphate is 2-6 tablets (100-300 mg) daily in divided doses as required.
Recommended parenteral dosage is Tiemonium Methylsulphate 2ml, 3 times daily, through intravenous or intramuscular route slowly.
SIDE EFFECT
Side effect with the use of Tiemonium Methylsulphate is very rare.
PRECAUTION
Caution should be taken for the treatment of patients with chronic bronchitis, coronary insufficiency, ambient hyperthermia, renal & hepatic insufficiency.
CONTRAINDICATION
Tiemonium Methylsulphate should not be used in cases of glaucoma, difficulty to urinate (disorders of prostate or bladder), tachycardia, myocardial infarction, paralytic ileus, pyloric stenosis and acute edema of the lung.
DRUG INTERACTION
Tiemonium Methylsulphate should not be used with other drugs without prior consult of a registered physician to avoid possible drug interaction.
Overdose: Occasional retention of urine may occur in excessive or overdoses.
USE IN PREGNANCY AND LACTATION
Tiemonium Methylsulphate may be used in pregnancy & lactating mother only if it is clearly needed by the assessment of risk benefit ratio.
STORAGE
Store tablet and injection at or below 30°C. Protect from light & moisture. Keep out of reach of children.
PACKAGING
TYVAS®: Tablet 50 mg: Each box contains 5 strips of 10 tablets in blister pack.
TYVAS®: Injection: Each box contains 3 strips of 2 ampoules of 2 ml in blister pack.